Cogentech is offering its diagnostic services for Hereditary Tumors (Cancer Risk) and for Personalized Medicine (Therapy) to clinicians, laboratories and hospitals.
The excellence of our diagnostic services is based on highly qualified staff, decennial experience, continuous formation and innovation, adherence to high quality standards and dedication to provide the best possible diagnostic service possible.
Timeline Genetic Testing Cogentech
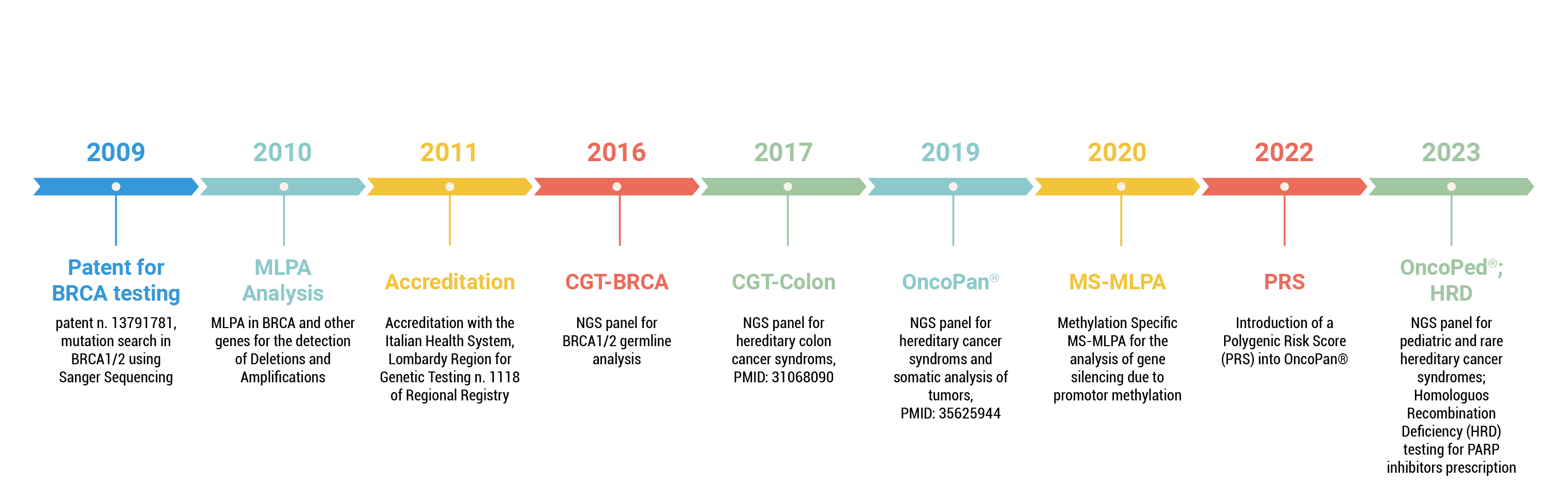
The CGT Lab has worked, first as a research group in IFOM and since 2011 in Cogentech SB Srl, as a diagnostic service lab in BRCA1 and BRCA2 mutation analysis. During this time CGT lab has provided more than 17.000 patients with a genetic testing result for cancer predisposition.
Cogentech has gradually increased his genetic testing service responding to an increasing demand, mainly for breast, ovarian and colorectal hereditary cancer diagnostics. Today we offer testing also for rare adult and pediatric hereditary cancers. In addition, we provide for interested clinicians a polygenic risk score (PRS) based on 313 single nucleotide polymorphisms (SNP) to refine the individual cancer risk evaluation.
Besides genetic testing for cancer risk, we offer also testing for personalized therapy in the context of mutations in the DNA repair pathway which renders patients eligible for specific drug treatments.
We continuously innovate and improve our portfolio thanks to intense interaction with our holding company, the cancer research institute IFOM ETS and with our clients.
For our services we use both proprietary solutions (OncoPan®, OncoPed®, OncoHRD, Sanger Sequencing) and commercial kits (MLPA, MS-MLPA) to provide the best diagnostic solutions.
We currently serve the main public and some private hospitals in the Lombardy region.
Click here to download the CGT Lab Service Charter
Click here to download the accredited examinations list
Click here to download the Public statement regarding the production and use of in-house devices by health care institutions
Click here for detailed list of Hereditary Cancer Syndromes and genes that are covered by OncoPan®
List of Hereditary Cancer Syndromes and genes that are covered by OncoPan®
| Cancer Syndrome |
Genes |
| Hereditary Breast / Ovaric Cancer (HBOC) |
ATM - BARD1 - BRCA1 - BRCA2 - BRIP1 - CDH1 - CHEK2 - PALB2 - PTEN - RAD51C - RAD51D - STK11 |
| Lynch syndrome |
EPCAM - MLH1 - MSH2 - MSH6 - PMS2 |
| Familial adenomatous polyposis (FAP) |
APC- MUTYH |
| Li-Fraumeni syndrome |
TP53 |
| Hereditary Diffuse Gastric Cancer |
CDH1 - CTNNA1 |
| Peutz-Jeghers syndrome |
STK11 |
| Cowden disease |
PTEN |
| Familial Melanoma |
ACD – BAP1 - CDKN2A - CDK4 - MC1R– MITF - POT1 -TERF21P- TERT |
| Juvenile Polyposis syndrome (JPS) |
BMPR1A - SMAD4 |
| Polymerase Proofreading Associated Polyposis (PPAP) |
POLE- POLD1 |
| Colorectal Cancer and Polyposis (rare hereditary conditions) |
AXIN2 - GREM1-MSH3 - NTHL1 - RNF43 |
| Hereditary Prostate Cancer |
ATM - BRCA1 - BRCA2 – CHECK2 - EPCAM - HOXB13 - MLH1 - MSH2 - MSH6 - PALB2 - PMS2 |
| Hereditary Pancreatic Cancer |
ATM - APC - BRCA1 - BRCA2 - CDKN2A - EPCAM - MLH1 - MSH2 - MSH6 - PALB2 - PMS2 – STK11 – TP53 |
| Polygenic Risk Score |
313 SNPs (for details see Mavaddat et al. Am. J. Hum. Genet.104:21-34) |
Click here for detailed list of the Pediatric and Rare Cancer Syndromes and genes that are covered by OncoPed®.
List of the Pediatric and Rare Cancer Syndromes and genes that are covered by OncoPed®
| Cancer Syndrome |
Genes |
| Lynch Syndrome |
EPCAM - MLH1 - MSH2 - MSH6 - PMS2 |
| Familial adenomatous polyposis (FAP) |
APC- MUTYH |
| Li-Fraumeni syndrome |
TP53 |
| Peutz-Jeghers syndrome |
STK11 |
| Cowden disease |
PTEN |
| Hereditary Pancreatic Cancer |
APC - BRCA1 - BRCA2 - CDKN2A - EPCAM - MLH1 - MSH2 - MSH6 - PALB2 - TP53 |
| DICER1 syndrome |
DICER1 |
| Gorlin syndrome (Nevoid basal cell carcinoma syndrome) |
PTCH1 - SUFU |
| Retinoblastoma |
RB1 |
| Carney Complex (CNC) |
PRKAR1A |
| Atypical teratoid/rhabdoid tumor (AT/RT) |
SMARCB1 - SMARCA4 |
| Von Hippel-Lindau syndrome (VHL) |
VHL |
| Wilms tumor |
WT1 – FBXW7 |
| Birt-Hogg-Dubé syndrome (BHD) |
FLCN |
| Hereditary papillary renal cell carcinoma (HPRCC) |
MET |
| Hereditary leiomyomatosis and renal cell cancer (HLRCC) |
FH |
| BAP1 tumor predisposition syndrome (BAP1-TPDS) |
BAP1 |
| POT1 tumor predisposition (POT1-TPD) |
POT1 |
Click here for detailed list of the Diagnostic Solutions for PARP inhibitor Therapy.
List of the Diagnostic Solutions for PARP inhibitor Therapy
| Diagnostic Application |
Genes |
| OncoPan |
BRCA1 - BRCA2 |
| OncoHRD |
BRCA1 - BRCA2 and Low Pass Whole Genome Sequencing for identification of HRD |
Click here for detailed list of the MLPA and MS-MLPA analysis we perform
List of the MLPA and MS-MLPA analysis we perform
| MLPA & MS-MLPA |
| APC SALSA MLPA® KIT (CE-IVD) |
| ATM1 SALSA MLPA® KIT(CE-IVD) |
| ATM2 SALSA MLPA® KIT(CE-IVD) |
| BARD1 SALSA MLPA®KIT (CE-IVD) |
| BRCA1 SALSA MLPA® KIT (CE-IVD) |
| BRCA2 SALSA® MLPA® KIT (CE-IVD) |
| BRCA2-CHEK2 SALSA MLPA® KIT (CE-IVD) |
| BRCA1-BRCA2-RAD51C SALSA® MS-MLPA® |
| CDH1 SALSA MLPA® KIT (CE-IVD) |
| CDKN2A/2B-CDK4 SALSA MLPA® KIT |
| CHEK2-ATM-TP53 SALSA MLPA® KIT(CE-IVD) |
| MLH1-MSH2 SALSA MLPA® KIT (CE-IVD) |
| MSH6-MUTYH SALSA MLPA® KIT(CE-IVD) |
| Mismatch Repair genes SALSA® MS-MLPA® (IVD) |
| PALB2-RAD50-RAD51C-RAD51D SALSA MLPA® KIT (CE-IVD) |
| PMS2 SALSA MLPA® KIT (CE-IVD) |
| PTEN SALSA MLPA® KIT(CE-IVD) |
| RB1 SALSA MLPA®KIT |
| SMAD4/BMPR1A-PTEN SALSA MLPA® KIT (CE-IVD) |
| SMARCB1 SALSA MLPA®KIT (CE-IVD) |
| STK11 SALSA MLPA® KIT (CE-IVD) |
| TP53 SALSA MLPA® KIT |
| additional analysis for genes included in the NGS panels can be provided upon request |
Vision
The company operates in the field of cancer molecular genetics and its aim is to develop diagnostic tools for the detection and quantification of particular germline genomic patterns that predispose to a risk of developing cancer. To do this, it has developed a proprietary
® instrument. The latter, in addition to diagnosing the presence of pathogenic variants of known genes predisposing to hereditary cancer syndromes, can be used to carry out population screening for hereditary-familial forms of breast/ovarian and colorectal cancer to intercept carriers of pathogenic germline variants of genes predisposing to these cancers and to assess with PRS (for breast cancer) the generic genetic risk for this cancer. Cogentech's values, as a Benefit Society, are represented by its commitment to pursuing aims of common benefit, set out in its articles of incorporation and certified in the annual 'impact report'.
Mission
We are a company with a Benefit C. format and as such committed to proposing solutions to problems that affect and have a significant social impact, such as those related to cancer diagnosis. We are on the market because we propose innovative and original solutions that respond to our strong Benefit C. vocation of investing in R&D, as dictated by our sole partner IFOM ETS. We also offer diagnostic solutions to niche problems commonly neglected by major commercial companies (e.g., pediatric cancers). The differences with our competitors are mainly attributable to the Benefit nature of the company, but above all to its controlling chain, the sole shareholder IFOM ETS Foundation and the AIRC charity that controls the latter. This 'virtuous circle' has Cogentech at its center, which on the one hand benefits from the innovation that IFOM's research can produce, on the other it responds to the need to provide concrete answers on the subject of cancer diagnostics, which the cancer patient, but also the
ordinary citizen expects from AIRC.
Contacts
We consider your complaints, if any, as a valuable tool of quality and, therefore, the starting point for stimulating actions to improve CGT Lab services and our relationship with the user.
You can submit any complaints by sending an e-mail to the following address:
gtic-service[@]ifom.eu